Covid 19 Lab Procedure Codes
HCPCS code C9803 Hospital outpatient clinic visit specimen collection for severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 coronavirus disease COVID-19 is intended to be reported when hospital staff perform specimen collection for COVID-19 testing. For more detailed guidance regarding diagnosis coding for COVID-19 and guidelines for pregnant patients see this CDC guideline.
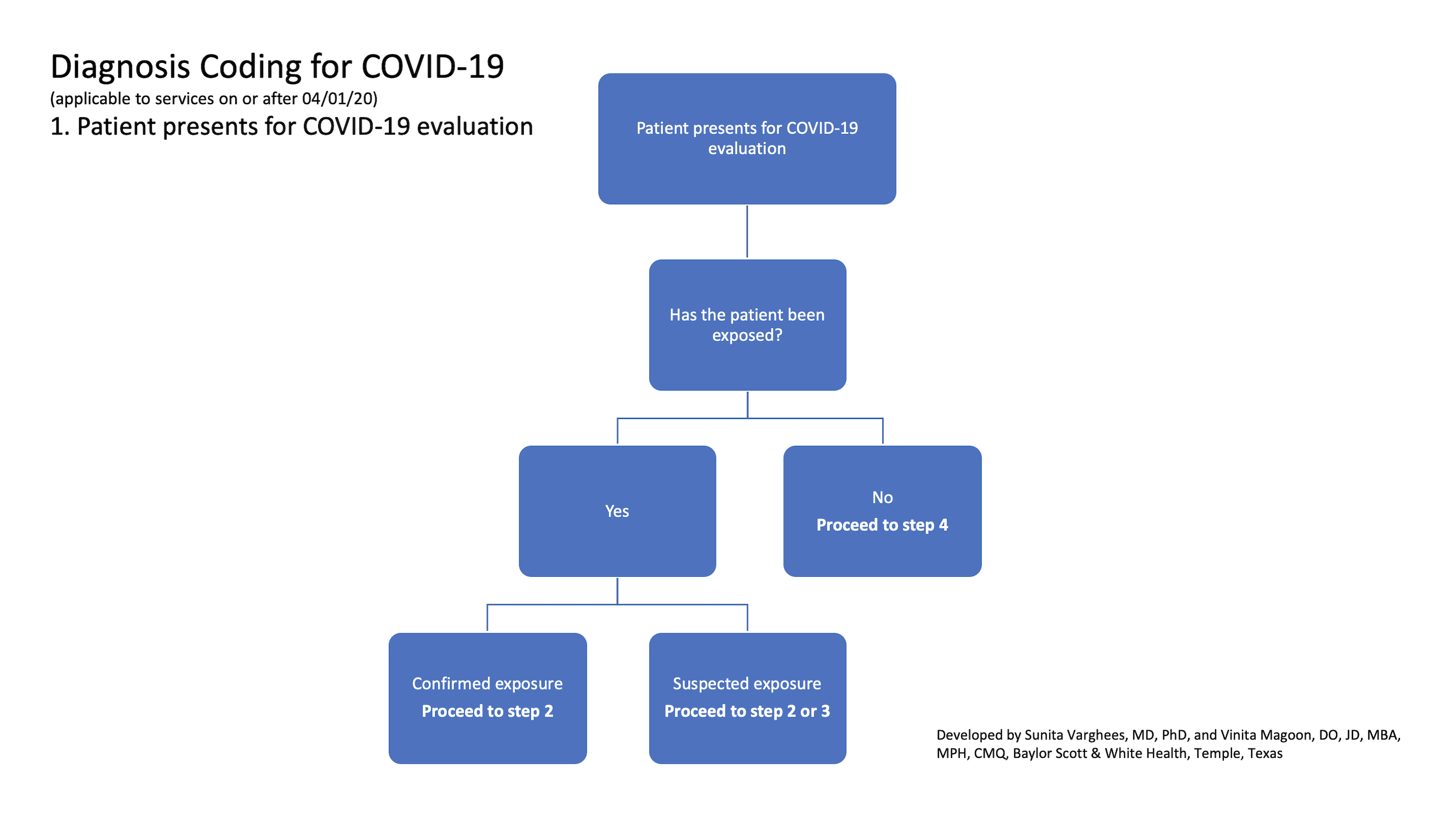
Covid 19 Diagnosis Coding Explained In A Flowchart Fpm
Also new and revised guidelines parenthetical notes and a new Appendix Q were added.

Covid 19 lab procedure codes. Assign code U071 COVID-19 for a confirmed diagnosis of the 2019 novel coronavirus disease COVID-19 as documented by the provider a positive COVID-19 test result or a presumptive positive COVID-19 test result. HCPCS Procedure Supply Codes U0002 - 2019-ncov coronavirus sars-cov-22019-ncov covid-19 any technique multiple types or subtypes includes all targets non-cdc The above description is abbreviated. Laboratory Code Long Descriptor Target 1.
G2023 specimen collection for severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 Coronavirus disease COVID-19 any specimen source G2024 specimen collection for severe acute respiratory syndrome coronavirus 2. Lower respiratory infection. It is essential that healthcare systems begin using these codes to.
This code is used specifically for CDC testing laboratories to test patients for SARS-CoV-2The second HCPCS Code U0002 announced on 5-Mar-2020 allows labs to bill for non-CDC laboratory tests for SARS-CoV-22019-nCoV COVID-19. Proper diagnosis is needed to represent the care provided and ensure we can identify and track the at -. Codes 91300 0001A and 0002A are used to report the Pfizer-BioNTech.
May 22 2020 Codes U0003U0004defined below are applicable to providers performing tests for the detection of SARSCoV2 or the diagnosis of the virus that causes COVID19 making use of high throughput equipment AND having the requisite CLIA license. Code 87635 is for the detection of SARS-CoV-2 COVID-19 and any pan-coronavirus types or subtypes and it can be reported with tests from multiple manufacturers using the stated technique. Codes for Coronavirus Lab Tests U0002 is a valid 2021 HCPCS code for 2019-ncov coronavirus sars-cov-22019-ncov covid-19 any technique multiple types or subtypes includes all targets non-cdc or just Covid-19 lab test non-cdc for short used in Diagnostic laboratory.
COVID -19 Related Codes U0001 CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel COVID-19 U0002 2019-nCoV Coronavirus SARS-CoV-22019-nCoV COVID-19 any technique multiple types or subtypes includes all targets non-CDC COVID-19. There are also two new HCPCS Level II testing codes for reporting Medicare beneficiaries being tested for COVID-19 and a new diagnosis code for reporting confirmed cases of COVID-19. When COVID-19 meets the definition of principal diagnosis code U071 COVID-19 should be sequenced first followed by the appropriate codes for associated manifestations except in the case of obstetrics patients as indicated in Section.
Use appropriate codes for the signs and symptoms eg R05 cough R0602 shortness of breath or R509 fever unspecified. If the COVID-19 is documented as being associated with a respiratory infection NOS it would be appropriate to assign. If the COVID-19 is documented as being associated with a lower respiratory infection not otherwise specified NOS or an acute respiratory infection NOS report with code J22 Unspecified acute lower respiratory infection with code B9729.
But those are for nucleic acid assays that detect multiple respiratory viruses in a multiplex reaction while CPT code 87635 is for the detection of SARS-CoV-2 COVID-19 and any pan-coronavirus types or subtypes. The first HCPCS Code U0001 can be used to bill for tests and to track new cases of the virus. Reporting HCPCS codes U0003U0004 for COVID-19 Lab Tests Date Issued.
Testing for the duration of the PHE for the COVID-19 pandemic. Which codes can you use for Coronavirus. These HCPCS codes are.
Severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 COVID-19 Do not use this procedure for Medicare members COVID-19 ICD-10 Coding. COVID-19 vaccine and 91301 0011A and 0012A are used to report the Moderna COVID-19 vaccine. Code 87635 does not require reporting of an additional CPT code for this service.
NCoV COVID-19 Use for Medicare members or Commercial members 87635 Infectious agent detection by DNA or RNA. The American Medical Association today released for immediate use Current Procedural Terminology codes for reporting on medical claims two laboratory tests 87636 and 87637 that simultaneously detect the COVID-19 virus influenza AB and respiratory syncytial virus.

Billing And Coding Guidelines Bcbsnd

Billing And Coding Guidelines Bcbsnd
Https Www Medica Com Media Documents Provider Covid 19 Testing Pdf La En Hash Ada9836ab9582b58d07613220b641038
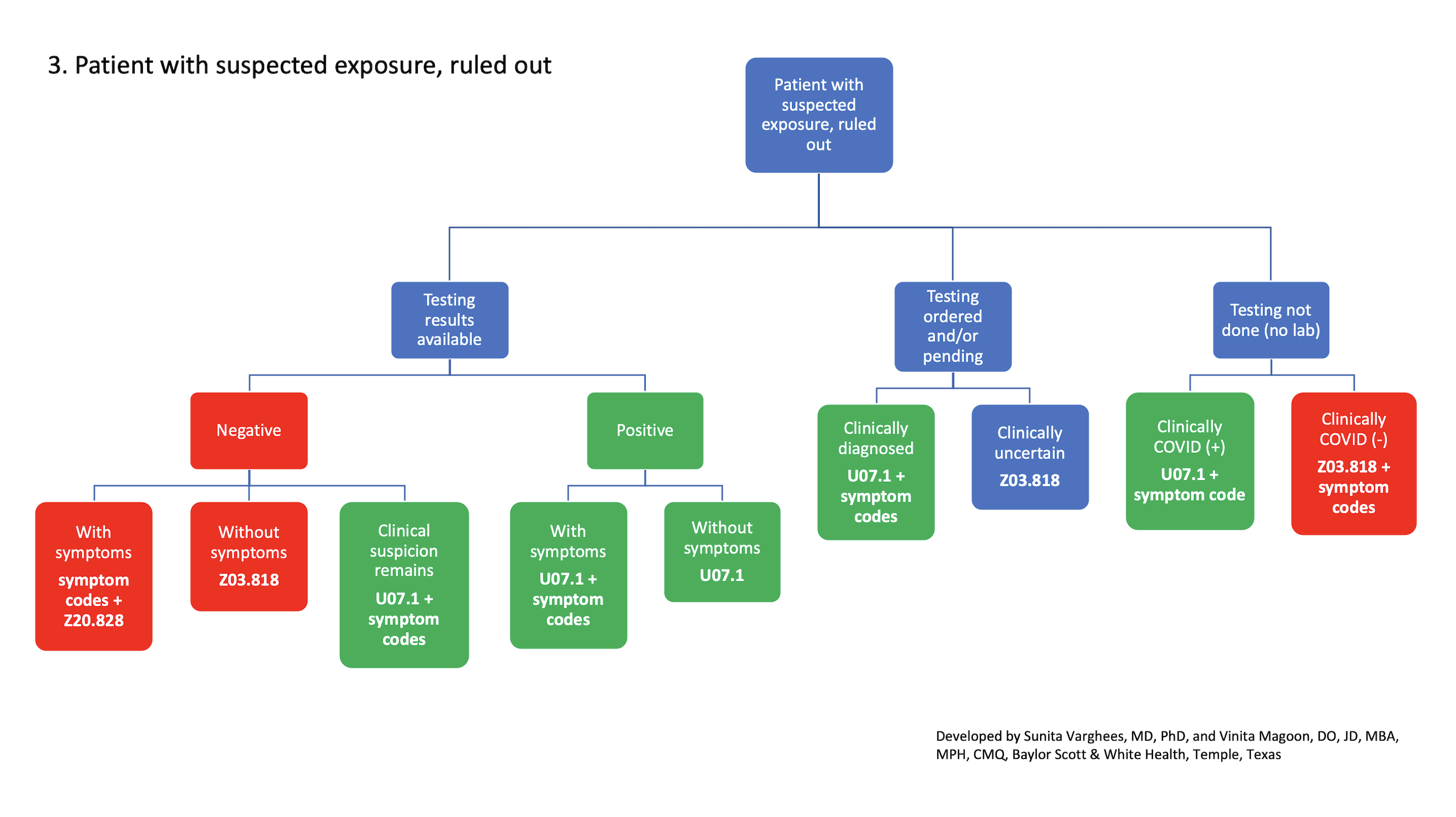
Covid 19 Diagnosis Coding Explained In A Flowchart Fpm

Telehealth And Covid 19 Billing And Coding Resources

Coding For Covid 19 Lab Testing And Specimen Collection Vitalware
/what-are-cpt-codes-2614950_final-fdf3a30069f242f9a37d41f131893d33.jpg)
An Overview Of Cpt Codes In Medical Billing

Telehealth And Covid 19 Billing And Coding Resources

Telehealth And Covid 19 Billing And Coding Resources

Posting Komentar untuk "Covid 19 Lab Procedure Codes"